
| Loan Amount | € 437,703 |
| Grant amount | € 65,655 |
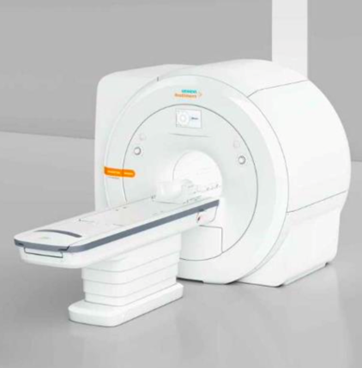
| Invested in | Siemens MAGNETOM Sempra |
| EU Directives met | Diagnostic quality, Health and Safety of workers and environmental protection |
The ‘Tesla Medical Center’ in Chernihiv was founded in 2007. The name
‘Tesla’ was chosen by the founder of the enterprise, paying homage to
Nikola Tesla, the brilliant scientist who discovered the magnetic fields, the
very foundation of Magnetic Resonance Diagnostics (MRI). MRI, CT and
ultrasound diagnostics were the focus of the clinics activities in the
beginning. Over time, the company developed into a full-fledged private
medical clinic. Today the Tesla Medical Center is the largest private clinic
in the Chernihiv region with four branches in the city of Chernihiv and one
large branch in the city of Nizhyn. Tesla Medical Center provides high
quality but affordable medical services for adults and children.
To improve the quality of its services to the community, the company built
a new 1,199 m2 medical center. The MRI department in the new center is
designed to cater for the access requirements of patients with limited
mobility, including bedridden patients.
With the help of a EU4Business-EBRD Credit Line loan and grant, the
company equipped the MIRI department with a new Siemens
MAGNETOM Sempra. The new MRI machine has superior energy
efficiency parameters and lower maintenance costs in comparison to the
old one. Especially the machine’s consumption of helium is significantly
lower than that of older models and will only require refilling every five
years.
Having thus strengthened its competitiveness in the domestic market, the
new machine also significantly improved the health and safety of the
doctors and nurses operating the equipment as well as that of patients
undergoing MRI diagnostics.
After the successful implementation and verification of this project, the
company will receive 15% of the loan value as a grant incentive, funded
under the EU4Business initiative of the European Union.
With the investment, the company now meets a wide variety of European
standards, including:
• Regulation (EU) 2017/745 of the European Parliament and of the
Council of 5 April 2017 on medical devices
• Directive 2014/30/EU of the European Parliament and of the
Council of 26 February 2014 on the harmonisation of the laws of
the Member States relating to electromagnetic compatibility
(recast) Text with EEA relevance
• Regulation (EU) 2016/425 of the European Parliament and of the
Council of 9 March 2016 on personal protective equipment and
repealing Council Directive 89/686/EEC (Text with EEA
relevance)